
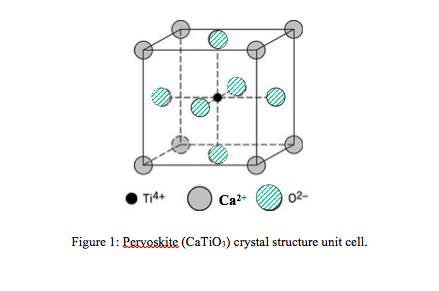
Calcium hydroxide, often known as slaked lime, is used in agriculture to neutralise acidic soil. Potassium present at the extreme left will have greater atomic radii of 0.227 nm while Co and Ni at the middle of period will have atomic radii of 0.125 nm each. As you go down the group the atomic radius increases. Potassium, Calcium, Cobalt and Nickle all are present in same period. It is the fifth most abundant element on earth. Hence, calcium has higher atomic radius than magnesium. It is a soft, silvery-white alkaline earth metal, whose name comes from the Latin word calx, which means lime. On moving down the group, the atomic radii increase as additional electron shells are added. This is because the number of protons and electrons increases but the the valence shell remain the same hence, causing more nuclear charge and more attraction between the valence electrons and nucleus. Calcium (Ca) is a chemical element of the periodic table, located in the group 2 and the period 4, and is having the atomic number 20. However, even for atoms of the same type, atomic radii can differ, depending on the oxidation state, the type of bonding.

Moving from left to right along the periods the atomic radii decreases.
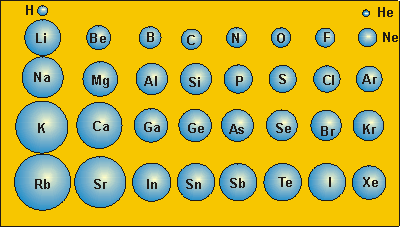
radius, as the elements are considered in order of increasing atomic number. Moving from top to bottom along the groups the atomic radii increases because as you move from top to bottom the number of shells increases hence, the distance of valence electrons from nucleus also increases and therefore, atomic radius increases. ii) K(19) is placed before Ca(20) in the same period (fourth period).Since the atomic radius decreases along a period,the atomic radius of calcium is. 1) nonmetallic properties and atomic radius. The trends in atomic radii are as follow, Using periodic table trends one can easily compare the atomic radii of elements.


 0 kommentar(er)
0 kommentar(er)
